24 Jan 2024
EpiEndo Pharmaceuticals celebrates milestones and achievements from the past 10 years
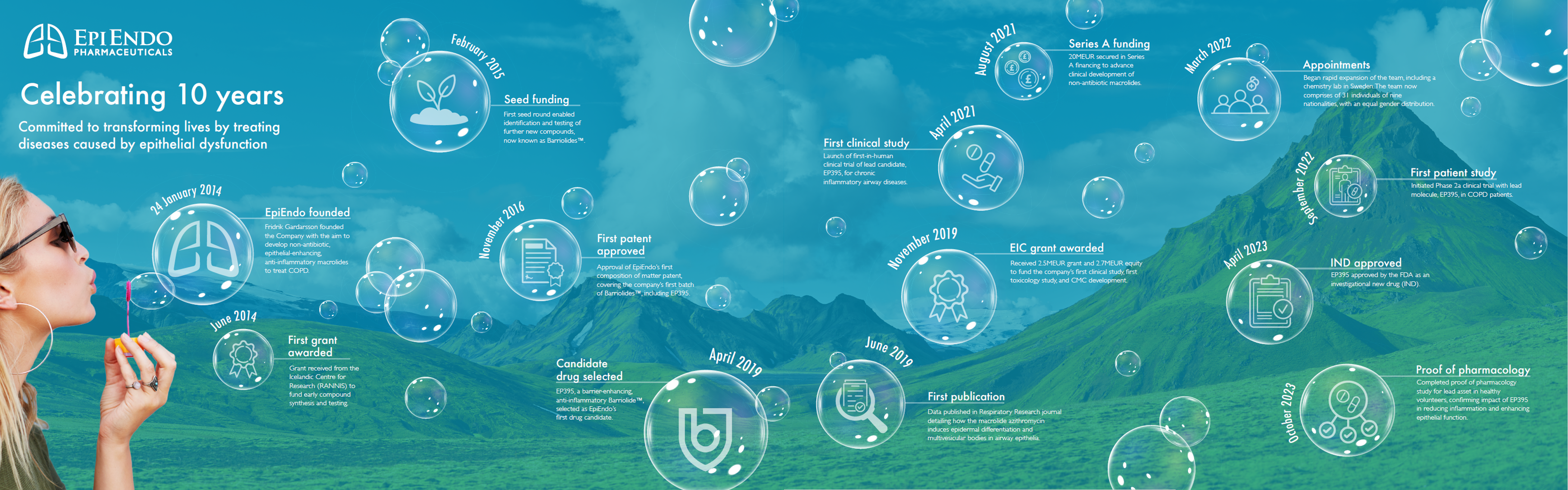
Over the past decade, the company has achieved a significant amount, from receiving its first grant, to selecting its first drug candidate, securing financing, and expanding the team.
A summary of our key achievements and important milestones are shown below.
- 24 January 2014 - Fridrik Gardarsson founded the Company with the aim to develop non-antibiotic, epithelial-enhancing, anti-inflammatory macrolides to treat Chronic Obstructive Pulmonary Disease (COPD).
- June 2014 – First grant received from the Icelandic Centre for Research (RANNIS) to fund early compound synthesis and testing.
- February 2015 - First seed round enabled identification and testing of further new compounds, now known as Barriolides™.
- November 2016 - Approval of EpiEndo’s first composition of matter patent, covering the company’s first batch of Barriolides™, including EP395.
- April 2019 - EP395, a barrier-enhancing, anti-inflammatory Barriolide™, selected as EpiEndo’s first drug candidate.
- June 2019 – First publication with data published in the Respiratory Research journal, which detailed how the macrolide azithromycin induces epidermal differentiation and multivesicular bodies in airway epithelia.
- November 2019 - Received 2.5MEUR grant and 2.7MEUR equity to fund the company’s first clinical study, first toxicology study, and CMC development.
- April 2021 - Launch of first-in-human clinical trial of lead candidate, EP395, for chronic inflammatory airway diseases.
- August 2021 - 20MEUR secured in Series A financing to advance clinical development of non-antibiotic macrolides.
- March 2022 - Began rapid expansion of the team, including a chemistry lab in Sweden. The team now comprises of 31 individuals of nine nationalities, with an equal gender distribution.
- September 2022 - Initiated Phase 2a clinical trial with lead molecule, EP395, in COPD patients.
- April 2023 - EP395 approved by the FDA as an investigational new drug (IND).
- October 2023 - Completed proof of pharmacology study for lead asset in healthy volunteers, confirming impact of EP395 in reducing inflammation and enhancing epithelial function.
Maria Bech, CEO, commented on this occasion, noting, “2024 is a particularly momentous year for EpiEndo. It’s been a decade now since our company was founded, with the clear mission of developing a new and innovative treatment to tackle COPD. We’ve remained dedicated to this goal over the years, and I want to thank the team for their work and contributions. We have achieved an enormous amount in a relatively short time, so I am excited to see what else the company will achieve in the years to come.”